what is the formula of the ionic compound expected to form between the elements br and k?
3.3 Formulas for Ionic Compounds
Learning Objectives
- Write the chemical formula for a simple ionic compound.
- Recognize polyatomic ions in chemical formulas.
We have already encountered some chemical formulas for simple ionic compounds. A chemical formulaA concise list of the elements in a compound and the ratios of these elements. is a concise listing of the elements in a compound and the ratios of these elements. To better sympathise what a chemic formula means, nosotros must consider how an ionic compound is constructed from its ions.
Ionic compounds exist every bit alternate positive and negative ions in regular, three-dimensional arrays called crystalsA three-dimensional assortment of alternating positive and negative ions. (Figure iii.6 "A Sodium Chloride Crystal"). As yous tin see, in that location are no individual NaCl "particles" in the array; instead, there is a continuous lattice of alternating sodium and chloride ions. However, we can use the ratio of sodium ions to chloride ions, expressed in the lowest possible whole numbers, as a way of describing the compound. In the case of sodium chloride, the ratio of sodium ions to chloride ions, expressed in lowest whole numbers, is 1:1, and then we use NaCl (one Na symbol and i Cl symbol) to represent the compound. Thus, NaCl is the chemical formula for sodium chloride, which is a concise manner of describing the relative number of different ions in the compound. A macroscopic sample is composed of myriads of NaCl pairs; each pair called a formula unit of measurementA prepare of oppositely charged ions that compose an ionic compound. . Although it is convenient to think that NaCl crystals are equanimous of individual NaCl units, Figure 3.6 "A Sodium Chloride Crystal" shows that no single ion is exclusively associated with any other single ion. Each ion is surrounded by ions of opposite charge.
Figure 3.half dozen A Sodium Chloride Crystal
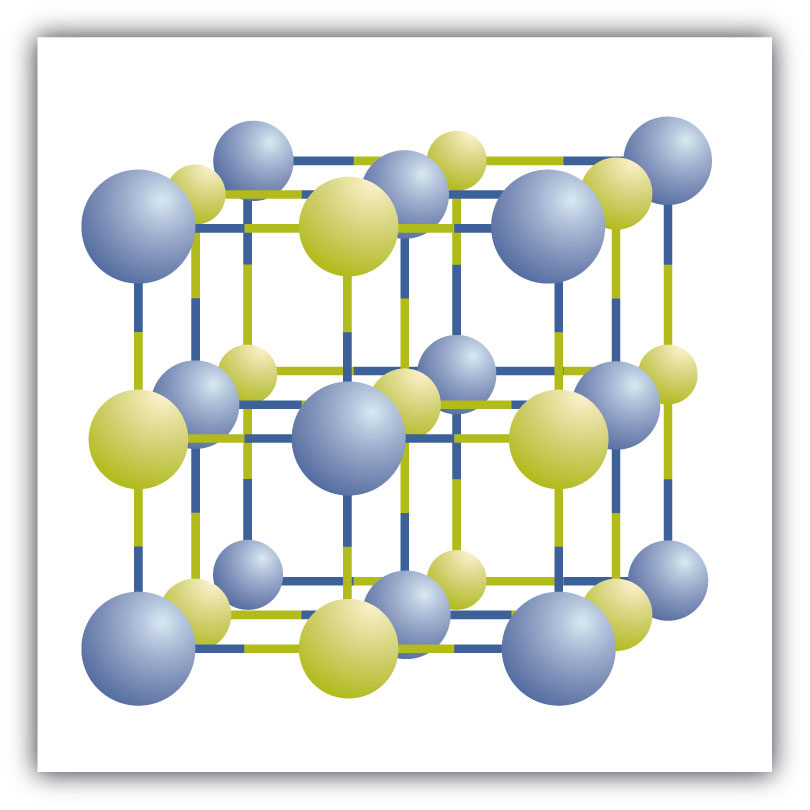
A crystal contains a iii-dimensional array of alternating positive and negative ions. The precise pattern depends on the chemical compound. A crystal of sodium chloride, shown here, is a drove of alternating sodium and chlorine ions.
Annotation
In Department 3.2 "Ions", nosotros encountered LiBr and MgO, which are formulas for other ionic compounds.
The formula for an ionic chemical compound follows several conventions. First, the cation is written earlier the anion. Because most metals form cations and most nonmetals form anions, formulas typically list the metal first then the nonmetal. Second, charges are not written in a formula. Remember that in an ionic compound, the component species are ions, non neutral atoms, fifty-fifty though the formula does not incorporate charges. Finally, the proper formula for an ionic compound ever obeys the post-obit dominion: the full positive accuse must equal the total negative charge. To determine the proper formula of whatever combination of ions, determine how many of each ion is needed to balance the full positive and negative charges in the chemical compound.
Notation
This dominion is ultimately based on the fact that affair is, overall, electrically neutral.
Annotation
Past convention, assume that at that place is only one atom if a subscript is non nowadays. We practice not use i as a subscript.
If we expect at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a i+ charge and the bromide ion has a one− charge. Only one ion of each is needed to balance these charges. The formula for lithium bromide is LiBr.
When an ionic chemical compound is formed from magnesium and oxygen, the magnesium ion has a 2+ accuse, and the oxygen atom has a 2− charge. Although both of these ions have college charges than the ions in lithium bromide, they still balance each other in a one-to-one ratio. Therefore, the proper formula for this ionic compound is MgO.
Now consider the ionic chemical compound formed by magnesium and chlorine. A magnesium ion has a two+ charge, while a chlorine ion has a 1− charge:
Mgtwo+ Cl−
Combining one ion of each does not completely balance the positive and negative charges. The easiest way to balance these charges is to assume the presence of two chloride ions for each magnesium ion:
Mg2+ Cl− Cl−
At present the positive and negative charges are balanced. We could write the chemical formula for this ionic compound as MgClCl, simply the convention is to use a numerical subscript when there is more than than i ion of a given type—MgCltwo. This chemical formula says that there are one magnesium ion and 2 chloride ions in this formula. (Do not read the "Cl2" part of the formula as a molecule of the diatomic elemental chlorine. Chlorine does not exist every bit a diatomic element in this chemical compound. Rather, information technology exists equally two individual chloride ions.) Past convention, the everyman whole number ratio is used in the formulas of ionic compounds. The formula Mg2Cl4 has balanced charges with the ions in a ane:two ratio, but it is not the lowest whole number ratio.
Note
Past convention, the everyman whole-number ratio of the ions is used in ionic formulas. At that place are exceptions for certain ions, such as Hg2 two+.
Case three
Write the chemical formula for an ionic compound composed of each pair of ions.
- the sodium ion and the sulfur ion
- the aluminum ion and the fluoride ion
- the 3+ iron ion and the oxygen ion
Solution
- To obtain a valence beat octet, sodium forms an ion with a 1+ charge, while the sulfur ion has a two− accuse. Ii sodium ane+ ions are needed to residue the 2− charge on the sulfur ion. Rather than writing the formula as NaNaS, we shorten it past convention to Na2S.
- The aluminum ion has a 3+ accuse, while the fluoride ion formed past fluorine has a 1− charge. Iii fluorine 1− ions are needed to remainder the 3+ charge on the aluminum ion. This combination is written equally AlF3.
- Iron tin can grade two possible ions, but the ion with a iii+ charge is specified hither. The oxygen atom has a ii− accuse every bit an ion. To balance the positive and negative charges, we look to the least common multiple—half-dozen: two iron iii+ ions will requite 6+, while three 2− oxygen ions will give vi−, thereby balancing the overall positive and negative charges. Thus, the formula for this ionic compound is FeiiO3.
Skill-Edifice Practice
-
the calcium ion and the oxygen ion
-
the 2+ copper ion and the sulfur ion
-
the 1+ copper ion and the sulfur ion
Write the chemical formula for an ionic chemical compound equanimous of each pair of ions.
Polyatomic Ions
Some ions consist of groups of atoms bonded together and have an overall electric charge. Because these ions contain more than one atom, they are called polyatomic ionsAn ion with more than one cantlet. . Polyatomic ions have characteristic formulas, names, and charges that should exist memorized. For example, NOiii − is the nitrate ion; information technology has one nitrogen atom and three oxygen atoms and an overall 1− charge. Table 3.1 "Some Polyatomic Ions" lists the nearly common polyatomic ions.
Table 3.one Some Polyatomic Ions
| Name | Formula |
|---|---|
| ammonium ion | NH4 + |
| acetate ion | C2H3O2 − (likewise written CH3COtwo −) |
| carbonate ion | COiii 2− |
| chromate ion | CrO4 2− |
| dichromate ion | Cr2O7 2− |
| hydrogen carbonate ion (bicarbonate ion) | HCO3 − |
| cyanide ion | CN− |
| hydroxide ion | OH− |
| nitrate ion | NO3 − |
| nitrite ion | NO2 − |
| permanganate ion | MnO4 − |
| phosphate ion | PO4 3− |
| hydrogen phosphate ion | HPO4 2− |
| dihydrogen phosphate ion | H2PO4 − |
| sulfate ion | SO4 2− |
| hydrogen sulfate ion (bisulfate ion) | HSOfour − |
| sulfite ion | SO3 2− |
The rule for constructing formulas for ionic compounds containing polyatomic ions is the same every bit for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. If more than than one of a particular polyatomic ion is needed to balance the charge, the entire formula for the polyatomic ion must be enclosed in parentheses, and the numerical subscript is placed outside the parentheses. This is to show that the subscript applies to the entire polyatomic ion. An example is Ba(NO3)ii.
Case iv
Write the chemical formula for an ionic compound equanimous of each pair of ions.
- the potassium ion and the sulfate ion
- the calcium ion and the nitrate ion
Solution
- Potassium ions have a charge of 1+, while sulfate ions have a accuse of ii−. We will need two potassium ions to residuum the charge on the sulfate ion, and then the proper chemic formula is KtwoThenfour.
- Calcium ions have a accuse of 2+, while nitrate ions have a charge of 1−. We volition need two nitrate ions to residue the charge on each calcium ion. The formula for nitrate must be enclosed in parentheses. Thus, we write Ca(NO3)ii as the formula for this ionic compound.
Skill-Building Exercise
-
the magnesium ion and the carbonate ion
-
the aluminum ion and the acetate ion
Write the chemical formula for an ionic compound composed of each pair of ions.
Recognizing Ionic Compounds
At that place are two ways to recognize ionic compounds. Beginning, compounds between metal and nonmetal elements are usually ionic. For example, CaBr2 contains a metallic chemical element (calcium, a group 2A metallic) and a nonmetallic element (bromine, a grouping 7A nonmetal). Therefore, it is most likely an ionic compound. (In fact, information technology is ionic.) In contrast, the compound NOii contains two elements that are both nonmetals (nitrogen, from group 5A, and oxygen, from group 6A). It is non an ionic chemical compound; information technology belongs to the category of covalent compounds that we will report in Chapter four "Covalent Bonding and Simple Molecular Compounds". Also annotation that this combination of nitrogen and oxygen has no electrical accuse specified, and so it is not the nitrite ion.
Second, if y'all recognize the formula of a polyatomic ion in a compound, the chemical compound is ionic. For example, if y'all run into the formula Ba(NO3)two, yous may recognize the "NOthree" part as the nitrate ion, NO3 −. (Recollect that the convention for writing formulas for ionic compounds is not to include the ionic accuse.) This is a inkling that the other part of the formula, Ba, is actually the Ba2+ ion, with the ii+ charge balancing the overall 2− charge from the two nitrate ions. Thus, this chemical compound is also ionic.
Example five
Identify each compound as ionic or not ionic.
- Na2O
- PCl3
- NHivCl
- OF2
Solution
- Sodium is a metal, and oxygen is a nonmetal; therefore, Na2O is expected to be ionic.
- Both phosphorus and chlorine are nonmetals. Therefore, PClthree is not ionic.
- The NHfour in the formula represents the ammonium ion, NH4 +, which indicates that this chemical compound is ionic.
- Both oxygen and fluorine are nonmetals. Therefore, OF2 is not ionic.
Skill-Building Practice
-
N2O
-
FeCl3
-
(NHfour)threePO4
-
SOCl2
Place each chemical compound as ionic or not ionic.
Looking Closer: Blood and Seawater
Science has long recognized that blood and seawater have like compositions. Later all, both liquids accept ionic compounds dissolved in them. The similarity may exist more than mere coincidence; many scientists think that the first forms of life on Earth arose in the oceans.
A closer wait, however, shows that blood and seawater are quite different. A 0.9% solution of sodium chloride approximates the common salt concentration found in blood. In contrast, seawater is principally a 3% sodium chloride solution, over 3 times the concentration in blood. Here is a comparing of the amounts of ions in blood and seawater:
| Ion | Percent in Seawater | Percent in Claret |
|---|---|---|
| Na+ | 2.36 | 0.322 |
| Cl− | 1.94 | 0.366 |
| Mg2+ | 0.13 | 0.002 |
| And soiv 2− | 0.09 | — |
| K+ | 0.04 | 0.016 |
| Catwo+ | 0.04 | 0.0096 |
| HCOthree − | 0.002 | 0.165 |
| HPO4 2−, H2POfour − | — | 0.01 |
Most ions are more abundant in seawater than they are in blood, with some important exceptions. There are far more hydrogen carbonate ions (HCOthree −) in blood than in seawater. This departure is significant considering the hydrogen carbonate ion and some related ions have a crucial role in controlling the acid-base properties of blood. (For more information on the acid-base properties of blood, see Affiliate 10 "Acids and Bases", Section 10.v "Buffers".) The amount of hydrogen phosphate ions—HPO4 2− and HiiPO4 −—in seawater is very low, just they are present in higher amounts in blood, where they also bear on acid-base properties. Another notable difference is that blood does not have significant amounts of the sulfate ion (And then4 ii−), but this ion is present in seawater.
Concept Review Exercises
-
What data is contained in the formula of an ionic compound?
-
Why do the chemic formulas for some ionic compounds incorporate subscripts, while others do not?
-
Write the chemic formula for the ionic chemical compound formed by each pair of ions.
- Mg2+ and I−
- Na+ and Oii−
Answers
-
the ratio of each kind of ion in the compound
-
Sometimes more than than one ion is needed to balance the charge on the other ion in an ionic compound.
-
- MgI2
- Na2O
Key Takeaways
- Proper chemical formulas for ionic compounds remainder the full positive charge with the total negative accuse.
- Groups of atoms with an overall accuse, called polyatomic ions, also exist.
Exercises
-
Write the chemical formula for the ionic chemical compound formed by each pair of ions.
- Na+ and Br−
- Mg2+ and Br−
- Mg2+ and S2−
-
Write the chemical formula for the ionic compound formed past each pair of ions.
- K+ and Cl−
- Mg2+ and Cl−
- Mg2+ and Se2−
-
Write the chemical formula for the ionic compound formed by each pair of ions.
- Na+ and N3−
- Mg2+ and Due norththree−
- Al3+ and S2−
-
Write the chemical formula for the ionic compound formed by each pair of ions.
- Li+ and Nthree−
- Mg2+ and P3−
- Li+ and P3−
-
Write the chemical formula for the ionic compound formed past each pair of ions.
- Feiii+ and Br−
- Iron2+ and Br−
- Au3+ and Stwo−
- Au+ and Due south2−
-
Write the chemical formula for the ionic chemical compound formed by each pair of ions.
- Crthree+ and O2−
- Cr2+ and O2−
- Atomic number 82two+ and Cl−
- Pb4+ and Cl−
-
Write the chemical formula for the ionic compound formed by each pair of ions.
- Cr3+ and NO3 −
- Atomic number 26ii+ and PO4 3−
- Ca2+ and CrO4 2−
- Al3+ and OH−
-
Write the chemical formula for the ionic compound formed by each pair of ions.
- NH4 + and NO3 −
- H+ and CriiO7 two−
- Cu+ and CO3 two−
- Na+ and HCOiii −
-
For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them.
- Ba and Southward
- Cs and I
-
For each pair of elements, determine the accuse for their ions and write the proper formula for the resulting ionic compound between them.
- Thou and South
- Sc and Br
-
Which compounds would you predict to exist ionic?
- Li2O
- (NHiv)twoO
- CO2
- FeSO3
- Chalf dozenH6
- C2HviO
-
Which compounds would you predict to exist ionic?
- Ba(OH)two
- CH2O
- NH2CONH2
- (NH4)twoCrO4
- C8H18
- NHiii
Answers
-
- NaBr
- MgBr2
- MgS
-
- Na3Northward
- Mg3N2
- Al2Sthree
-
- FeBr3
- FeBr2
- AuiiS3
- Au2S
-
- Cr(NOthree)3
- Fethree (POiv)ii
- CaCrOfour
- Al(OH)iii
-
- Ba2+, Due south2−, BaS
- Cs+, I−, CsI
-
- ionic
- ionic
- not ionic
- ionic
- non ionic
- not ionic
wilsonjuspencesses.blogspot.com
Source: https://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/s06-03-formulas-for-ionic-compounds.html
0 Response to "what is the formula of the ionic compound expected to form between the elements br and k?"
Post a Comment